Given the transformative changes in consumer viewing and their impact on advertising, brands are increasingly relying on social influencers to promote their products. Such promotions can be very successful, because they are made in a seemingly informal manner and appear significantly more genuine than a straight advertisement. While the posts are typically drafted by the influencers, where there is a material connection between the influencer and the brand, brands must monitor such posts to confirm that the third-party posts contain claims that are consistent with what the brand can properly make. The recent posting by Kim Kardashian about Diclegis morning sickness medicine illustrates this point.
Perhaps no one is more talented in getting a message out than Kim Kardashian, whose Instagram account alone consists of over 3,000 posts with over 48 million followers. Even a quick review of Kim’s Instagram Account (@KimKardashian) reveals that she does more than post provocative selfies or pictures of her daughter or ever-present family. Indeed, her account is frequently used to promote Kardashian products, as well as products from third-party sponsors.
Ms. Kardashian is once again pregnant. To help address her morning sickness this time around, she evidently relied on the prescription drug Diclegis, which is an FDA approved drug for the treatment of nausea and vomiting in pregnant women who do not respond to conservative management. Ms. Kardashian has been so thrilled with the results that she made the following post on her Instagram account:
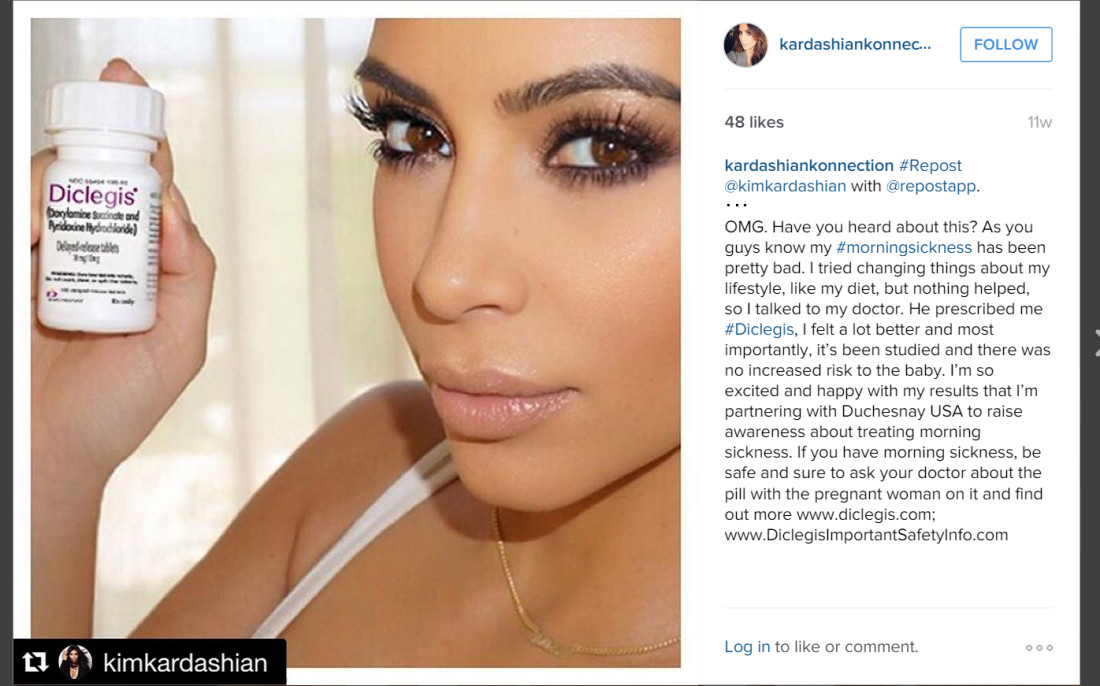
Even though the post appeared only on @KimKardashian social media pages, and not on the brand’s page, the United States Food and Drug Administration (FDA) issued a Warning Letter to its distributor Duchesnay, Inc. challenging the post as false and calling it misleading advertising that failed to include appropriate risk information required in traditional advertising. the FDA treated the social media post as misbranding the drug.
For example, the FDA found that the social media post “entirely omits all risk information.” The FDA was not persuaded that Kim’s statement “find out more www.diclegis.com; www.DiclegisImportantSafetyInfo.com,” which appears at the end of the social media post was sufficient because it fails to provide material information about the consequences that may result from the use of the drug and suggests that it is safer than has been demonstrated. The FDA also objected to the social media post on grounds that it failed to provide material information regarding the drug’s full approved indications, including important limitations of use.
The FDA demanded that the social media post be immediately discontinued and that the manufacturer submit a comprehensive plan of action to disseminate corrective advertising. To that end, Kim provided a lengthy Instagram post containing the corrective statements.
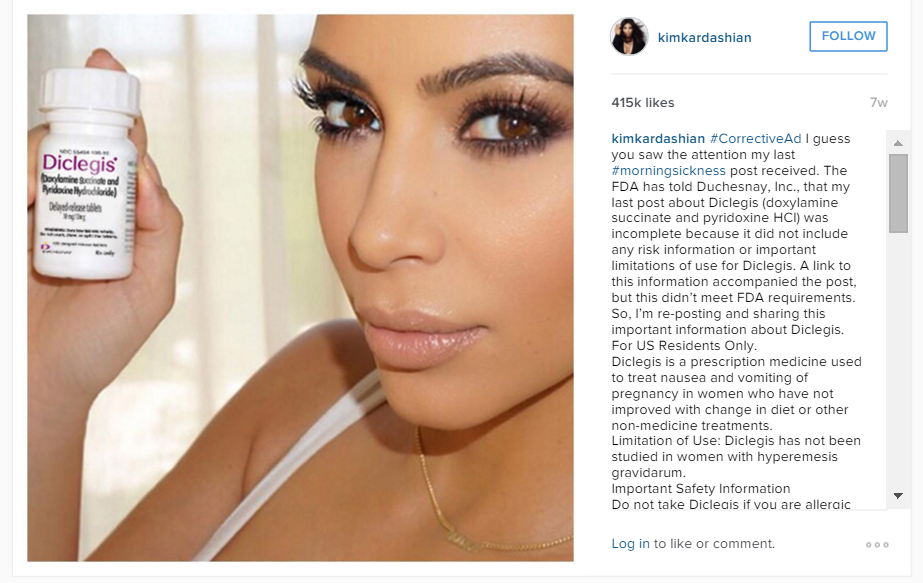
The FDA’s Warning Letter sends a strong message to brands that they must actively monitor the posts by social media influencers, particularly where there is a business relationship. Brands must be vigilant and confirm that the influencer is not making a claim or statement that the brand could not make itself or without including additional disclosures.
The FDA’s Warning Letter is particularly interesting given that it's the Federal Trade Commission that has been mostly in the headlines with respect to social media compliance obligations. Drug marketers, however, should keep in mind that the FDA has also issued Guidance for social media platforms for advertising prescription drugs and medical devices. Like the FTC guidelines, the FDA takes the position that if the space constraints of a particular platform preclude a proper disclosure, the marketer should reconsider using that particular platform.
Take Away: Brands must monitor what social media influencers are saying about their products and confirm that the claims are consistent with what the brand could permissibly state in its own advertising.
- Partner
Marketers, advertisers, agencies and suppliers, among others, regularly seek Andy’s counsel regarding legal aspects of their advertising and promotional marketing businesses. He’s pragmatic and always looks for ...





